The human body has sophisticated defenses against the deposition of calcium minerals that stiffen heart tissues, researchers at the University of Illinois Urbana-Champaign and collaborators at UCLA Health and the University of Texas at Austin found in a new study that provides the first detailed, step-by-step documentation of how calcification progresses.
“Heart disease is the leading killer annually—about 18 million deaths per year—and that number is growing. A large proportion is the result of calcification,” said study leader Bruce Fouke, a U. of I. professor of earth science and environmental change and director of the Roy J. Carver Biotechnology Center at Illinois.
“When the aortic valve calcifies, an ultra-invasive surgery to replace the valve is the only option currently. That heightens the urgency to get a handle on this process to more effectively move forward with drug development and testing.”
The aortic valve is the portal through which oxygenated blood gets pumped out of the heart to the body, opening and closing more than 3 billion times during the average lifespan. Calcium deposits can grow within the three tissue flaps that make up the valve, called leaflets, stiffening them and leaving them unable to open all the way.
“With calcification in blood vessels, a stent can help, but you can’t do that with the aortic valve. Every organ in the body can be in perfect condition, but if the aortic valve stops functioning, that’s the end of that life,” said Mayandi Sivaguru, the first author of the paper and the director of the Cytometry and Microscopy to Omics Facility in the Carver Biotechnology Center.
Despite the prevalence and biological importance, little is known about how the calcium deposits form or grow. Fouke’s group has pioneered the field of “GeoBioMed,” a combination of geology, biology and medicine, and previously applied it to the study of kidney stones.
In a new study, titled “Osteopontin stabilization and collagen containment slows amorphous calcium phosphate transformation during human aortic valve leaflet calcification,” published in the journal Scientific Reports, Fouke’s group at Illinois teamed up with colleagues at the UCLA School of Medicine and the Jackson School of Geoscience at Texas to study and document the steps of calcium deposit formation in aortic valves from human cadaver hearts.
“We used more than 12 modalities of study, including optical microscopy, electron microscopy and spectroscopy, to investigate the nature and progression of mineralization and protein localization in the aortic valve for the first time. This multimodal analysis set us apart, uncovering a new line of evidence to better understand cardiovascular calcification,” Sivaguru said.
The starting point is healthy leaflet tissue. Then tiny spherules of calcium phosphate form in the smooth muscle layer of the leaflets.
Crucially, the team found that the form of calcium phosphate in the mineral deposits is not the same type as found in bone, called apatite, as has been widely thought. Instead, the deposits consist predominately of amorphous calcium phosphate, which has the ability to morphologically shapeshift and atomically rearrange.
As they grow, the spherules coalesce into layers that encrust and stiffen the collagen and smooth muscle fibers that give the leaflets their flexibility. These processes combine to form large nodules that rotate, touch each other and further stiffen the tissues.
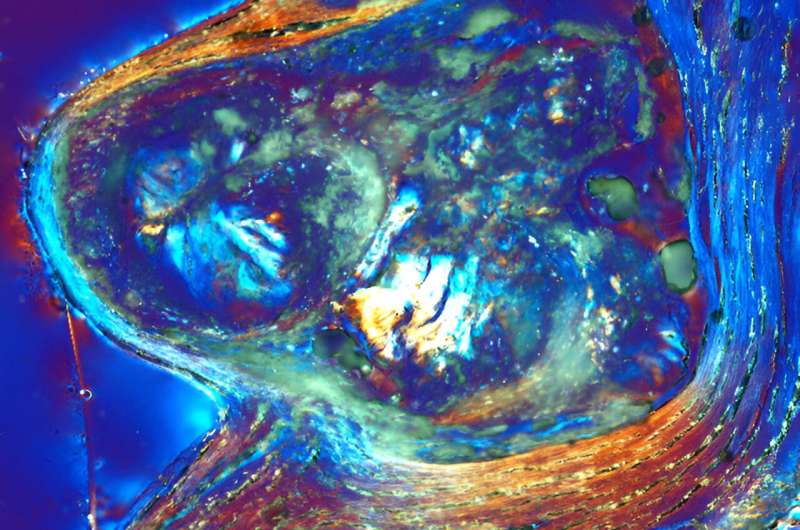
“Immediately, we saw that the reactions within the tissues of the valves were virtually identical to those we’ve studied in coral reefs, hot springs and many other natural environments that harbor life-water-mineral interactions,” said Fouke, who is the Ralph E. Grim Professor at Illinois.
“Our blood is saturated with calcium and phosphate. Calcification of collagen and growth of nodules is inevitable given our blood chemistry, biology and composition.
“However, the silver lining to all of this is that we also found that our body has evolved these incredibly intricate and effective processes to fight mineralization. It can’t stop it, but it can slow it down dramatically.”
The researchers found two defense mechanisms. As the tiny ACP spherules form and begin to coalesce, the heart tissues produce large amounts of the protein osteopontin. Osteopontin promotes apatite growth and calcification in bones and kidney stones, so the findings initially puzzled the researchers, Fouke said. But osteopontin has the opposite, inhibitory effect on ACP, slowing down collagen calcification and nodule aggregation.
“That’s why knowing it’s ACP instead of apatite is so important. Enhancing the release of osteopontin could be an important new target to slow down calcification to a level that it won’t be a threat or require surgical intervention,” Fouke said.
The body’s second defense is the very collagen where the nodules form. The researchers found that as the nodules start growing, the collagen fibers stretch around and contain them, forming a water barrier that further slows nodule growth.
In addition to investigating possible therapeutic applications of osteopontin to slow calcification, the researchers also hope their work opens new avenues of investigation into preventing initial growth and dissolving already-formed mineral deposits throughout the human body.
In collaboration with Mayo Clinic, the team is now applying their multimodal GeoBioMed approach to studying calcification in human breast tumors, a hallmark of the disease.
More information:
Mayandi Sivaguru et al, Osteopontin stabilization and collagen containment slows amorphous calcium phosphate transformation during human aortic valve leaflet calcification, Scientific Reports (2024). DOI: 10.1038/s41598-024-62962-8
Citation:
Study defines the process of and defenses against cardiac valve calcification (2024, June 27)
retrieved 27 June 2024
from https://medicalxpress.com/news/2024-06-defenses-cardiac-valve-calcification.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.

