Seminoma, a type of testicular cancer that predominantly affects young men, is the most common testicular germ cell tumor and is characterized by its similarity to primordial germ cells (PGCs), the precursor to sperm cells that form early in embryonic development.
Despite a generally favorable prognosis with high cure rates through chemotherapy, approximately 10% of seminoma cases exhibit resistance, leading to metastasis and challenging relapses.
Research on seminoma to develop better treatments has been impeded by the lack of relevant mouse models and the scarcity of seminoma samples. Additionally, the tumor itself is complex, with varied cell types and a significant presence of immune cells, making it hard to analyze in bulk.
“These obstacles hinder comprehensively understanding seminoma’s genetic and molecular underpinnings,” says Kotaro Sasaki, a physician scientist at the University of Pennsylvania’s School of Veterinary Medicine who is leading the charge on researching the biology of urogenital cancers at the molecular level.
In a paper published in the journal Cell Reports, a team led by Sasaki and collaborators developed the first in vitro seminoma model and presented the most comprehensive single-cell atlas of human male germ cell development reported to date, shedding light on chromosomal anomalies and signaling pathways that may contribute to seminoma development.
“Our study, which used samples from various stages of early human male development, has provided key insights,” Sasaki says. “We now better understand the origins, chromosomal anomalies, and signaling pathways involved. And most excitingly, we have developed the first in vitro seminoma model, a vital step toward understanding and potentially treating the disease.”
Sasaki and his team first used single-cell RNA sequencing to analyze the gene expression profiles of normal male germ cells at various stages of development by using tissue samples, which enabled the researchers to develop the most comprehensive map detailing the trajectory from migrating PGCs to differentiated spermatozoa precursors and highlighted key transitions and gene expression changes.
“In comparing seminoma cells to this developmental trajectory,” Sasaki says, “it was found that seminoma closely resembles premigratory/migratory PGCs.”
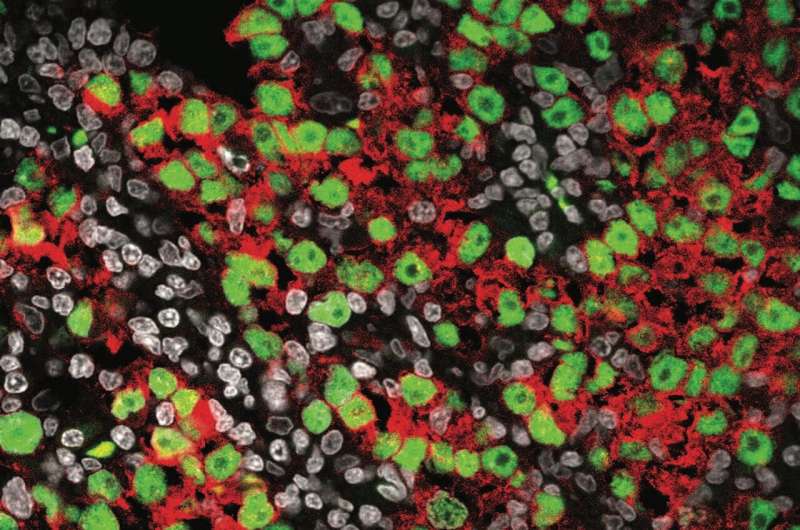
He explains that germ cells are formed early in the first trimester of pregnancy as PGCs, and after that, they travel from the place of birth to the testis through the process called migration. The team’s data suggest that seminoma most closely resembles PGCs before or during migration phase, and this resemblance suggests that seminoma originates from these early-stage germ cells.
The researchers discovered the presence of isochromosome 12p in seminoma cells, which is an aberration involving an extra copy of the short arm of chromosome 12.
Sasaki says this chromosomal anomaly is present in as much as 90% of seminomas and includes genes crucial for both germ cell development and tumorigenesis, such as DPPA3, NANOG, and KRAS.
Sasaki’s team developed their in vitro seminoma model using skin cells reprogrammed to an embryonic-like state—allowing them to re-differentiate into primordial germ cell–like cells—from tissue samples from people with Pallister-Killian syndrome, a rare genetic disorder characterized by the presence of an extra 12p.
Using this new seminoma model along with fresh seminoma single-cell RNA sequencing data, the team gleaned key insights into the altered signaling pathways in seminoma cells, like how apoptosis (cell death), angiogenesis (formation of new blood vessels), and MAPK/ERK signaling (involved in cell growth and survival) are significantly upregulated in seminoma cells.
“We found that the MAPK/ERK pathway, in particular, is significantly activated in seminoma cells, which could be key to understanding their proliferation and resistance mechanisms,” Sasaki says.
Looking ahead, Sasaki’s research will focus on refining the in vitro seminoma model by introducing specific mutations known to be involved in seminoma, such as activating mutations in the KIT gene, which drives the RAS/MAPK signaling pathway.
More information:
Keren Cheng et al, Defining the cellular origin of seminoma by transcriptional and epigenetic mapping to the normal human germline, Cell Reports (2024). DOI: 10.1016/j.celrep.2024.114323
Citation:
First in vitro seminoma model unveils the genetics of testicular cancer (2024, June 19)
retrieved 19 June 2024
from https://medicalxpress.com/news/2024-06-vitro-seminoma-unveils-genetics-testicular.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.

