A treatment paradox has recently come to light in prostate cancer: Blocking testosterone production halts tumor growth in early disease, while elevating the hormone can delay disease progression in patients whose disease has advanced.
The inability to understand how different levels of the same hormone can drive different effects in prostate tumors has been an impediment to the development of new therapeutics that exploit this biology.
Now, a Duke Cancer Institute-led study, performed in the laboratory of Donald McDonnell, Ph.D. and appearing in Nature Communications, provides the needed answers to this puzzle.
The researchers found that prostate cancer cells are hardwired with a system that allows them to proliferate when the levels of testosterone are very low. But when hormone levels are elevated to resemble those present in the normal prostate, the cancer cells differentiate.
“For decades, the goal of endocrine therapy in prostate cancer has been to achieve absolute inhibition of androgen receptor function, the protein that senses testosterone levels,” said lead investigator Rachid Safi, Ph.D., research assistant professor in the Department of Pharmacology and Cancer Biology, at Duke University School of Medicine.
“It’s been a highly effective strategy, leading to substantial improvements in overall survival,” he said. “Unfortunately, most patients with advanced, metastatic disease who are treated with drugs to inhibit androgen signaling will progress to an aggressive form of the disease for which there are limited therapeutic options.”
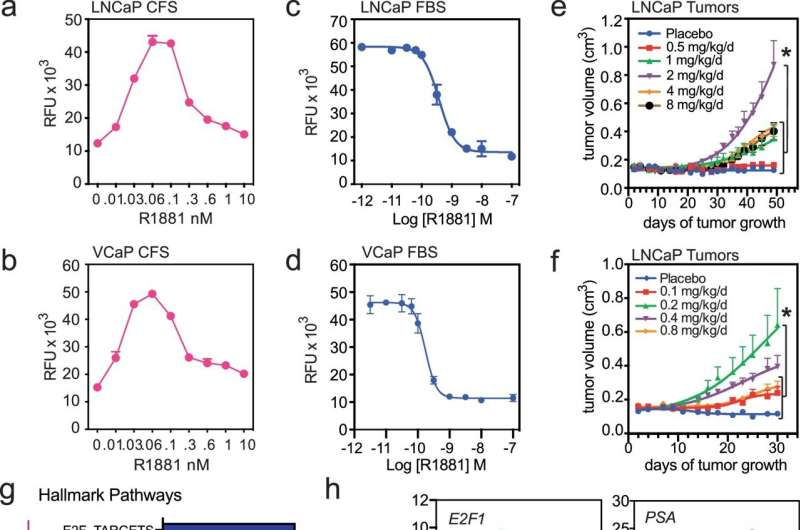
Using a combination of genetic, biochemical, and chemical approaches, the research team defined the mechanisms that enable prostate cancer cells to recognize and respond differently to varying levels of testosterone, the most common androgenic hormone.
It turned out to be rather simple. When androgen levels are low, the androgen receptor is encouraged to “go solo” in the cell. In doing so, it activates the pathways that cause cancer cells to grow and spread. However, as androgens rise, the androgen receptors are forced to “hang out as a couple,” creating a form of the receptor that halts tumor growth.
“Nature has designed a system where low doses of hormones stimulate cancer cell proliferation and high doses cause differentiation and suppress growth, enabling the same hormone to perform diverse functions,” McDonnell said.
In recent years, clinicians have begun treating patients with late-stage, therapy resistant prostate cancers using a monthly, high-dose injection of testosterone in a technique called bi-polar androgen therapy, or BAT. The inability to understand how this intervention works has hindered its widespread adoption as a mainstream therapeutic approach for prostate cancer patients.
“Our study describes how BAT and like approaches work and could help physicians select patients who are most likely to respond to this intervention,” McDonnell said. “We have already developed new drugs that exploit this new mechanism and are bringing these to the clinic for evaluation as prostate cancer therapeutics.”
In addition to McDonnell and Safi, study authors include Suzanne E. Wardell, Paige Watkinson, Xiaodi Qin, Marissa Lee, Sunghee Park, Taylor Krebs, Emma L. Dolan, Adam Blattler, Toshiya Tsuji, Surendra Nayak, Marwa Khater, Celia Fontanillo, Madeline A. Newlin, Megan L. Kirkland, Yingtian Xie, Henry Long, Emma Fink, Sean W. Fanning, Scott Runyon, Myles Brown, Shuichan Xu, Kouros Owzar, and John D. Norris.
More information:
Rachid Safi et al, Androgen receptor monomers and dimers regulate opposing biological processes in prostate cancer cells, Nature Communications (2024). DOI: 10.1038/s41467-024-52032-y
Citation:
Study solves testosterone’s paradoxical effects in prostate cancer (2024, September 4)
retrieved 4 September 2024
from https://medicalxpress.com/news/2024-09-testosterone-paradoxical-effects-prostate-cancer.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.

