Neurogenetic disorders, such as neurofibromatosis type 1 (NF1), are diseases caused by a defect in one or more genes, which can sometimes result in cognitive and motor impairments. Better understanding the neural underpinning of these disorders and how they affect motor and cognitive abilities could contribute to the development of new treatment strategies.
Researchers at Stanford University and Washington University School of Medicine recently performed a study on mice aimed at investigating the impact of Nf1 gene mutations, which cause the NF1 neurogenetic disorder, on oligodendroglial plasticity, an adaptive brain process known to contribute to cognitive and motor functions.
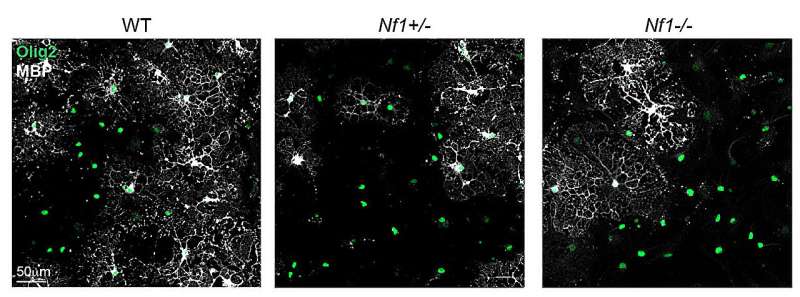
Their findings, published in Nature Neuroscience, provide strong evidence that Nf1 mutations delay the development of oligodendroglia, a type of glial cells that support the functioning of the central nervous system, causing disruptions in motor learning.
“This study started nearly a decade ago, when the concept that a glial cell type called an oligodendrocyte that forms myelin—the fatty substance that insulates axons and regulates the speed of neural signal conduction—can exhibit neuronal activity-mediated (experience-dependent) plasticity was newly appreciated,” Yuan Pan, David H. Gutmann and Michelle Monje-Deisseroth, co-authors of the paper, told Medical Xpress.
“At that time, studies from several groups, including Dr. Monje’s, demonstrated that oligodendroglial precursor cells (OPCs) respond to neuronal firing (activity) and this process supports the generation of new oligodendrocytes and adaptive changes in myelination.”
Oligodendroglial plasticity has been found to support the healthy functioning of the brain, contributing to learning, as well as attention and memory. The Gutmann and Monje laboratories at Stanford and Washington University have been investigating the neural processes underlying the NF1 neurogenetic syndrome for some time, as part of a long-standing research collaboration.
“NF1 is a neurogenetic syndrome caused by mutations in the Nf1 gene,” Pan, Gutmann and Monje said. “Children and adults with NF1 are prone to learning difficulties and glial tumors (gliomas).
“Our findings about the roles for this kind of neuroplasticity in healthy brain function raised the question of whether oligodendroglial plasticity may be impaired in NF1, a condition in which children often have deficits in neurological functions, like motor learning, that depend on oligodendroglial plasticity.”
As part of their recent study, Gutmann, Monje and their colleagues sought to determine if and how Nf1 mutations dysregulated oligodendroglial plasticity. To do this, they analyzed a diverse collection of new genetically engineered mouse strains, including some with Nf1 mutations only in oligodendrocyte precursor cells (OPCs) and others harboring patient-derived germline Nf1 gene mutations.
“We used optogenetics and the complex wheel test (motor learning experience) to modulate neuronal activity, and then measured oligodendroglial responses to neuronal activity,” Pan, Gutmann and Monje explained. “We also used the complex wheel test to examine motor learning in Nf1-mutant mice and cellular/molecular approaches to understand the underlying mechanisms.”
The researchers found that Nf1 mutation impaired the ability of individual OPCs to differentiate into mature and myelinating oligodendrocytes. This observation could provide interesting insight about the underpinnings of the motor disturbances seen in children with NF1.
First, Pan, Gutmann, Monje and their colleagues found that the deficit in OPC differentiation observed in mice with Nf1 mutations resulted in the delayed development of oligodendroglia. Notably, this pattern is consistent with that observed in the brains of children with NF1, which presented delayed myelin development.
“The observed loss of oligodendroglial plasticity and related motor learning deficits helps explain why children with NF1 often exhibit learning difficulties,” Pan, Gutmann and Monje said. “Another surprising and important observation is that heterozygous Nf1-mutant mice exhibit random focal OPC hyperdensities, which are reminiscent of the T2 hyperintensities (now called focal areas of signal intensity; FASI) detected in children with NF1.
“We found that the location and size of these OPC hyperdensities correlated with performance in motor learning.”
The researchers’ experiments also demonstrated that some, but not all, NF1 patient-derived Nf1 germline mutations can cause focal OPC hyperdensities in the brain. These hyperdensities reflect the activation of an understudied signaling pathway that could be potentially targeted by future treatments.
“Our findings provide a new understanding of the contributors (oligodendroglial plasticity) to neurological problems experienced by individuals with NF1 and suggest exciting opportunities for new treatment strategies,” Pan, Gutmann and Monje added.
“Our next studies will aim to understand how we can rescue the oligodendroglial dysfunction in NF1, by targeting candidates identified in this work.”
More information:
Yuan Pan et al, Nf1 mutation disrupts activity-dependent oligodendroglial plasticity and motor learning in mice, Nature Neuroscience (2024). DOI: 10.1038/s41593-024-01654-y
© 2024 Science X Network
Citation:
Nf1 gene mutations disrupt brain cell plasticity and motor learning in mice (2024, June 26)
retrieved 26 June 2024
from https://medicalxpress.com/news/2024-06-nf1-gene-mutations-disrupt-brain.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.

