Researchers are exploring a new potential avenue for pancreatitis treatment: autophagy, a cellular recycling process. Autophagy helps maintain healthy pancreatic acinar cells by removing damaged organelles like mitochondria and the endoplasmic reticulum (ER).
A new review published in eGastroenterology highlights the link between defective autophagy and pancreatitis. Impaired autophagy contributes to pancreatitis by allowing damaged organelles to accumulate within acinar cells. This accumulation disrupts cellular function and can ultimately lead to cell death.
“Autophagy plays a vital role in keeping pancreatic acinar cells healthy,” explains Dr. Wen-Xing Ding, lead author of the study. “When autophagy is defective, damaged organelles build up, stressing the cells and potentially contributing to pancreatitis.”
The review also explores the role of mitochondrial dysfunction and lysosomal dysfunction in pancreatitis. Mitochondria are the cell’s powerhouses, and lysosomes are the compartments responsible for breaking down cellular waste. Both mitochondrial damage and impaired lysosomal function are observed in pancreatitis, further hindering the cell’s ability to clear out damaged components.
The research team identified several potential therapeutic targets within the autophagy pathway. VMP1, a protein involved in both autophagy and ER-phagy (removal of damaged ER), is downregulated in pancreatitis. TFEB, a master regulator of lysosomal biogenesis and autophagy, is also decreased. Additionally, alcohol consumption disrupts autophagy by increasing ATG4B, a protease that regulates LC3-II, a key autophagy protein.
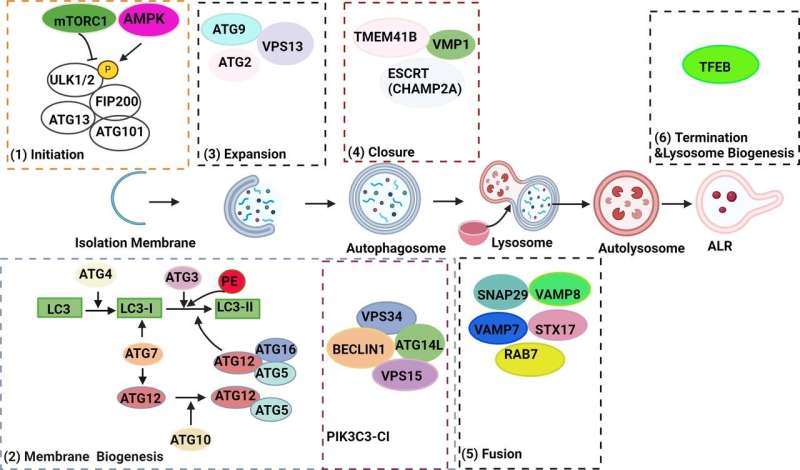
“Animal studies suggest that manipulating these proteins can influence the severity of pancreatitis,” Dr. Ding says. “Upregulating VMP1 or TFEB, or downregulating ATG4B, enhances autophagy and protects against pancreatitis.”
While further research is needed, targeting autophagy may offer a novel therapeutic approach for pancreatitis. Future studies will focus on the specific role of autophagy receptors in targeting damaged organelles during pancreatitis and the potential for manipulating autophagy to treat the disease in humans.
More information:
Wen-Xing Ding et al, Recent insights about autophagy in pancreatitis, eGastroenterology (2024). DOI: 10.1136/egastro-2023-100057
Provided by
First Hospital of Jilin University
Citation:
The link between defective autophagy and pancreatitis could point to new treatments (2024, May 24)
retrieved 24 May 2024
from https://medicalxpress.com/news/2024-05-link-defective-autophagy-pancreatitis-treatments.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.

